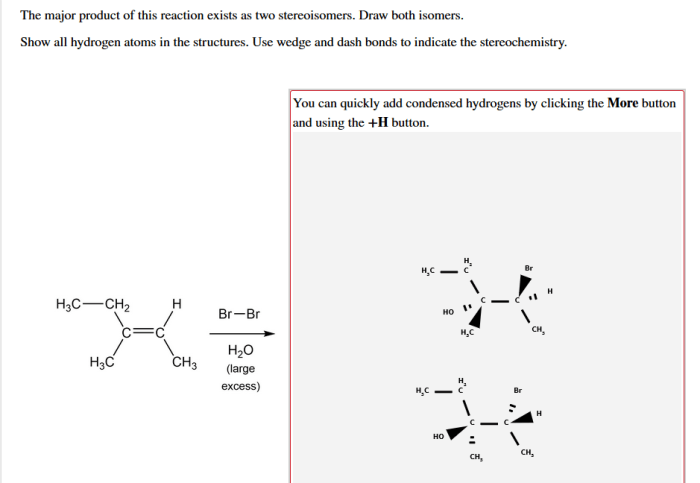
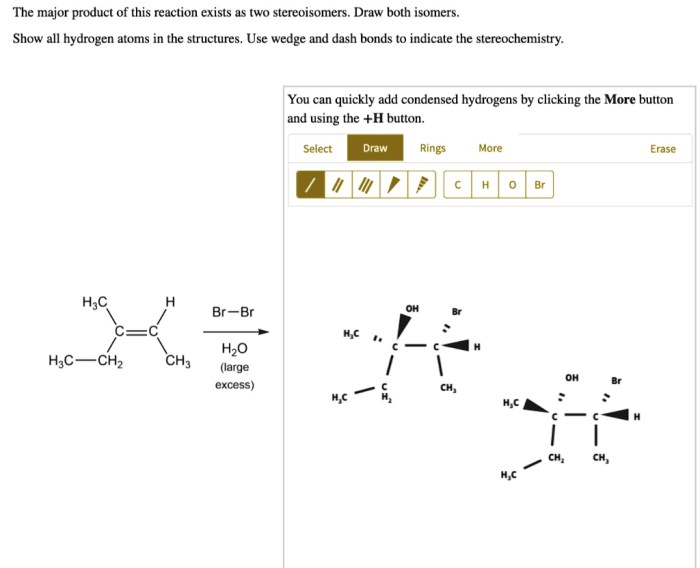
The major product of this reaction exists as two stereoisomers, a concept that delves into the fascinating realm of chirality and the intricate world of stereoisomerism. Stereoisomers, molecules with the same molecular formula but distinct spatial arrangements of atoms, play a pivotal role in various scientific disciplines, from pharmaceuticals to materials science.
This discourse will explore the captivating world of stereoisomers, unraveling the mechanisms that govern their formation, deciphering their unique properties, and showcasing their profound impact on biological systems and technological applications.
Stereochemistry and Stereoisomers: The Major Product Of This Reaction Exists As Two Stereoisomers
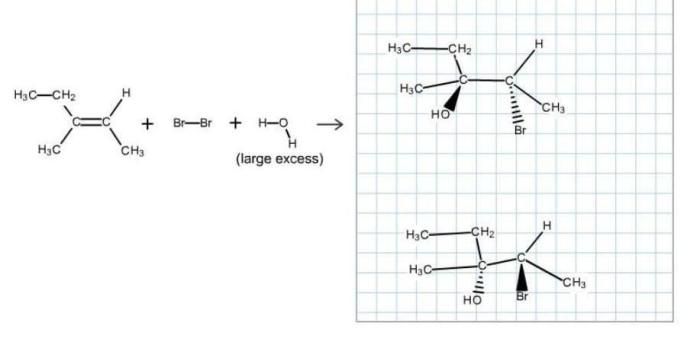
Stereochemistry deals with the spatial arrangement of atoms within molecules. Stereoisomers are molecules that have the same molecular formula but differ in the spatial arrangement of their atoms.
Chirality is a property of molecules that lack a plane of symmetry. Chiral molecules exist as two non-superimposable mirror images, known as enantiomers.
Diastereomers are stereoisomers that are not enantiomers. They have the same molecular formula and connectivity but differ in the spatial arrangement of their atoms.
Formation of Stereoisomers in Reactions
Stereoisomers can be formed in reactions through various mechanisms, such as nucleophilic addition, electrophilic addition, and substitution reactions.
The stereochemistry of the reactants and reagents can influence the stereochemistry of the products. For example, in nucleophilic addition reactions, the nucleophile can add to the electrophile from either side, resulting in the formation of two enantiomeric products.
In electrophilic addition reactions, the electrophile can add to the double bond from either face, resulting in the formation of two diastereomeric products.
Properties and Separation of Stereoisomers
Stereoisomers can have different physical and chemical properties. For example, enantiomers have identical physical properties but differ in their interactions with chiral molecules.
Diastereomers can have different physical and chemical properties, including different melting points, boiling points, and reactivities.
Stereoisomers can be separated using various techniques, such as chromatography and crystallization. Chromatography is a technique that separates molecules based on their different interactions with a stationary and mobile phase.
Crystallization is a technique that separates molecules based on their different solubilities in a solvent.
Biological Significance of Stereoisomers
Stereoisomers play a crucial role in biological systems. Many biological molecules, such as proteins and nucleic acids, are chiral.
The different biological activities of stereoisomers are known as stereoisomerism. For example, one enantiomer of a drug may be effective, while the other enantiomer may be inactive or even harmful.
Stereoisomerism is also important in enzyme specificity. Enzymes are proteins that catalyze specific chemical reactions. The active site of an enzyme is chiral and can only bind to one enantiomer of a substrate.
Applications of Stereoisomers, The major product of this reaction exists as two stereoisomers
Stereoisomers have a wide range of applications in various fields, including pharmaceuticals, materials science, and food chemistry.
In pharmaceuticals, stereoisomers are used to develop drugs with specific biological activities. For example, the drug thalidomide was originally marketed as a sedative but was later found to cause birth defects in children whose mothers took the drug during pregnancy.
In materials science, stereoisomers are used to develop polymers with specific properties. For example, the polymer polylactic acid (PLA) is a biodegradable plastic that is made from the enantiomer of lactic acid.
In food chemistry, stereoisomers are used to develop flavors and fragrances. For example, the flavor of the strawberry is due to the enantiomer of 2-methylbutanal.
FAQs
What are stereoisomers?
Stereoisomers are molecules with the same molecular formula but different spatial arrangements of atoms.
How do stereoisomers form?
Stereoisomers can form when a reaction creates a new chiral center or changes the configuration of an existing chiral center.
What are the different types of stereoisomers?
The two main types of stereoisomers are enantiomers and diastereomers. Enantiomers are mirror images of each other, while diastereomers are not.
What is the significance of stereoisomers?
Stereoisomers can have different physical and chemical properties, which can affect their biological activity and other applications.