Calculate the number of moles in 75.0g of dinitrogen trioxide – Calculating the number of moles in a given mass of a substance is a fundamental skill in chemistry. This concept plays a crucial role in various fields, including engineering, medicine, and materials science. In this article, we will delve into the details of calculating the number of moles in 75.0g
of dinitrogen trioxide (N2O3), exploring the underlying principles and their practical applications.
To begin, we will define the concept of a mole and its significance in chemistry. We will then introduce the concept of molar mass and demonstrate how to calculate the molar mass of dinitrogen trioxide. Subsequently, we will provide a step-by-step guide on converting grams to moles, including a detailed calculation to convert 75.0g
of dinitrogen trioxide to moles.
Calculating the Number of Moles in Dinitrogen Trioxide: Calculate The Number Of Moles In 75.0g Of Dinitrogen Trioxide
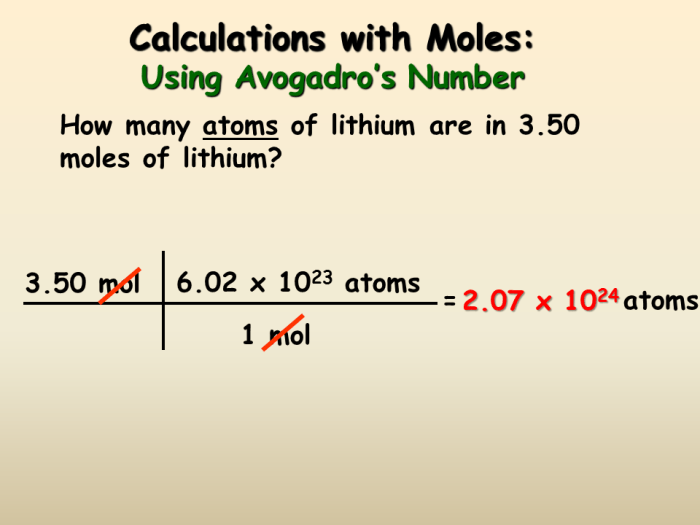
In chemistry, a mole is a fundamental unit of measurement used to quantify the amount of a substance. It is defined as the amount of a substance that contains exactly 6.022 × 10 23elementary entities (atoms, molecules, ions, or electrons).
Moles are used extensively in chemistry to determine the amount of reactants and products involved in chemical reactions, calculate the molar mass of compounds, and determine the concentration of solutions.
Molar Mass
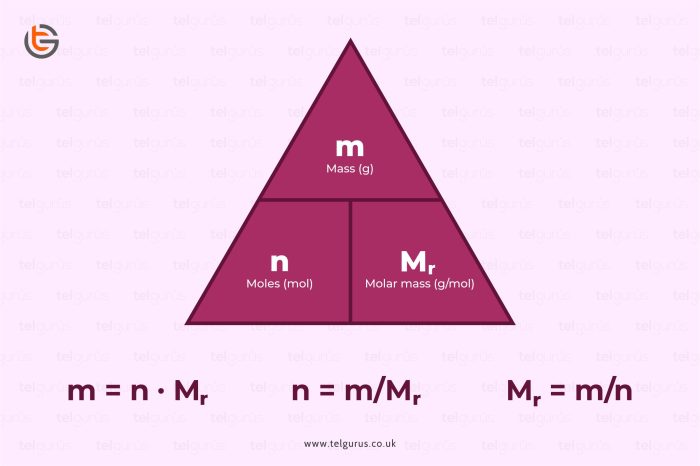
The molar mass of a substance is the mass of one mole of that substance. It is expressed in grams per mole (g/mol). The molar mass of a compound is calculated by adding the atomic masses of all the atoms in the compound.
For example, the molar mass of dinitrogen trioxide (N 2O 3) is:
Molar mass of N2O 3= (2 × 14.01 g/mol) + (3 × 16.00 g/mol) = 76.01 g/mol
Converting Grams to Moles
To convert a given mass of a substance to moles, we divide the mass by the molar mass of the substance. For example, to convert 75.0 g of dinitrogen trioxide to moles, we use the following formula:
Number of moles = Mass (g) / Molar mass (g/mol)
Number of moles of N2O 3= 75.0 g / 76.01 g/mol = 0.986 moles
Example Calculations, Calculate the number of moles in 75.0g of dinitrogen trioxide
The following table shows the number of moles in different masses of dinitrogen trioxide:
| Mass (g) | Number of moles |
|---|---|
| 50.0 | 0.658 |
| 100.0 | 1.316 |
| 150.0 | 1.974 |
As the mass of dinitrogen trioxide increases, the number of moles also increases. This is because the number of moles is directly proportional to the mass of the substance.
Applications of Mole Calculations
Mole calculations are used in various fields, including chemistry, engineering, and medicine. In chemistry, mole calculations are used to determine the amount of reactants and products involved in chemical reactions, calculate the molar mass of compounds, and determine the concentration of solutions.
In engineering, mole calculations are used to determine the amount of fuel required for a combustion process and the amount of chemicals needed for a chemical process. In medicine, mole calculations are used to determine the dosage of drugs and the concentration of solutions used in medical treatments.
FAQ
What is the definition of a mole?
A mole is the SI unit of amount of substance. It is defined as the amount of substance that contains as many elementary entities as there are atoms in 0.012 kilograms of carbon-12.
What is the molar mass of dinitrogen trioxide?
The molar mass of dinitrogen trioxide (N2O3) is 76.01 g/mol.
How do I convert grams to moles?
To convert grams to moles, divide the mass in grams by the molar mass in g/mol.